
The proper calculation of electrostatic lattice constants has to consider the crystallographic point groups of ionic lattice sites for instance, dipole moments may only arise on polar lattice sites, i. These concepts require the determination of higher order Madelung constants or so-called electrostatic lattice constants. The electrostatic interaction model of ions in solids has thus been extended to a point multipole concept that also includes higher multipole moments like dipoles, quadrupoles etc. Accordingly, the Madelung constant only represents the monopole-monopole term.
Equation of lattice energy of nacl series#
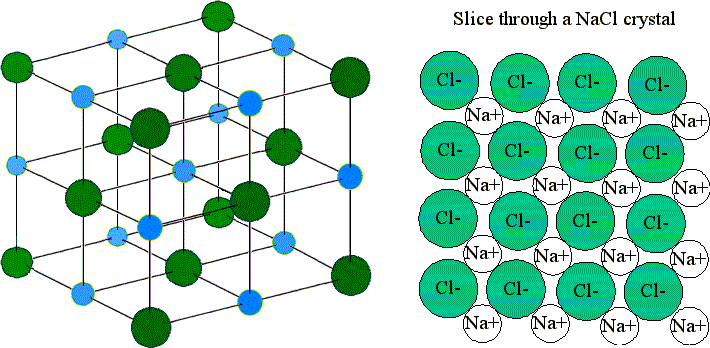
It is shown by electrostatics that the interaction between two point charges only accounts for the first term of a general Taylor series describing the interaction between two charge distributions of arbitrary shape. multipole moments of the charge density might be required. In particular cases, however, when the ions reside on lattice site of certain crystallographic point groups, the inclusion of higher order moments, i.e. This is allowed, if the electron distribution of the ion is spherically symmetric. It is assumed for the calculation of Madelung constants that an ion's charge density may be approximated by a point charge. The Madelung constant allows for the calculation of the electric potential V i of all ions of the lattice felt by the ion at position r i V i = e 4 π ε 0 ∑ j ≠ i z j r i j Generalization The energy required to break these bonds for one mole of an ionic solid under standard conditions is the lattice energy. This energy must be given to the system in order to break the anion–cation bonds. īecause the anions and cations in an ionic solid attract each other by virtue of their opposing charges, separating the ions requires a certain amount of energy. It is named after Erwin Madelung, a German physicist. The Madelung constant is used in determining the electrostatic potential of a single ion in a crystal by approximating the ions by point charges.

Note that in this case, the sum is divergent, but there are methods for summing it which give a converging series. Each number designates the order in which it is summed. The Madelung constant being calculated for the NaCl ion labeled 0 in the expanding spheres method.


 0 kommentar(er)
0 kommentar(er)
